Clinical Development Strategy, Planning & Operations Support
Our seasoned pharmaceutical physicians and functional specialists work to ensure that your program is on the optimal path forward, balancing risk and value, while responding to changes in the external environment. Alacrita's core team leverages 150+ industry-experienced, clinical development strategy and operations consultants, with backgrounds spanning a broad range of therapeutic areas and product modalities. Our extensive consulting resources allow us to offer you versatile, fit-for-purpose expertise that can be tailored to the exact needs of your clinical development program, enabling us to support you in each crucial area from planning and strategy to execution and tactical support.
Clinical Development Strategy & Clinical Development Plans
- Developing target product profiles
- Clinical development strategy
- Clinical development plans
- Developing study synopses and trial protocols
- Clinical trials benchmarking
- Disease indication strategy and lifecycle management
- Statistical Analysis Plans and Biostatistics Support
Clinical Operations
- Clinical operations support (CRO and site management)
- Resolving recruitment issues
- Clinical Quality Assurance and cGCP compliance
Regulatory
- Defining regulatory strategy and optimal pathways
- ODD, FTD, Breakthrough, Priority Review applications
- IND and NDA/MAA/BLA readiness analysis
- Providing strategic or hands-on support for interacting with the FDA and EMA
- Preparing dossiers and submissions
- Click here for full details on our RA capabilities
Alacrita has a number of CMO-level clinical MDs in our consulting network, who support our biotech clients as interim Chief Medical Officers, during for example, periods of extended search for a full-time individual. These Alacrita consultants are capable of overseeing flagship clinical trials, guiding a portfolio of programs, providing leadership to junior medics in the organization, as well as representing the company with the board of directors, investors and regulatory authorities.
Why Alacrita?
Our drug development consultants each bring an average of 20 years of industry experience and wisdom to our clients' program teams. Many have made significant contributions to the development of launched products and have held global responsibilities for marketed medicines. With Alacrita, you can access high-caliber, clinical development experts with proven track records and precisely relevant expertise. Whether you need to rapidly augment your existing capabilities, move past a development or regulatory hurdle with minimal delay, or obtain high-level strategic guidance, we have the experience and personnel to help you.
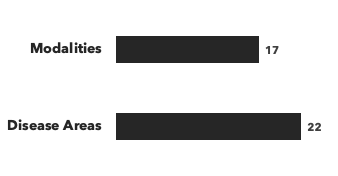
Number of drug development consultants by education, and number of modalities/disease areas covered: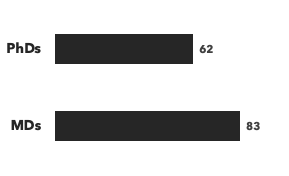
- Antibodies, ADCs
- Biomarkers
- Cell Therapy
- Diagnostics
- Discovery Platforms
- DNA/RNA Drugs
- Drug Delivery Tech.
- Gene Therapy
- Imaging Tools
- Medical Devices
- Medtech Software
- Microbiome Drugs
- Oncolytic Virus
- Peptides/Protein Drugs
- Radiopharmaceuticals
- Small molecules
- Vaccines
- Autoimmune/Immunology
- Cardiovascular
- Central Nervous System
- Congenital Disorders
- Dermatology/Aesthetics
- Digestive/GI
- Ear
- Endocrine and Metabolic
- Fibrosis
- Genitourinary Diseases
- Haematology
- Infectious Disease
- Inflammation/Pain
- Injuries and Trauma
- Musculoskeletal
- Non-medical
- Oncology
- Ophthalmology
- Orphan Diseases
- Respiratory
- Urology (non-onc)
- Women’s Health
Recent Clinical Development Projects:
- Interim Chief Medical Officer for fibrosis company: A well-capitalized, private biotech company with a drug discovery platform identified multiple first-in-class and best-in-class compounds targeting critical pathways widely involved in inflammatory and fibrotic diseases. As lead compounds progressed toward the development phase, the company was considering a broad spectrum of disorders as initial and follow on targets for therapy, including liver fibrotic disorders, idiopathic pulmonary fibrosis, renal fibrosis, and inflammatory bowel disease. The company desired a chief medical officer (CMO) with deep development experience in the broad area of fibrotic disease including liver, gastrointestinal, renal, and lung disease and organizational experience in the roles and responsibilities of a CMO. An Alacrita CMO with substantial fibrotic expertise was engaged by the company.
- Clinical development support for first-in-class pediatric oncology drug: For a listed US biotech company with marketed products, Alacrita's pediatric oncologist consultant provided ongoing support with Phase I/II design, protocol development and medical oversight of a first-in-class oncology drug for a pediatric population. The drug mechanism has an immunotherapy modality and continues to be investigated in clinical trials.
- Providing clinical and medical support in immuno-oncology: A leading immuno-oncology biotech company with a pipeline of novel, first-in-class clinical stage assets needed medical support for multiple clinical and medical affairs activities in Europe and the United States. Our support included providing advice and assistance with the following:
-
- Clinical development strategy and execution of the clinical development plans for the company’s drug candidates
- Drug safety and pharmacovigilance
- Fostering existing and future relationships with key opinion leaders, medical advisors and medical advisory boards.
- We also assessed portfolio expansion opportunities and other corporate development activities from a clinical development and medical perspective.
-
- Regulatory pathway for new wound care products: A world-leading manufacturer of wound care products was assessing opportunities in advanced wound care and had mapped regulatory pathways relating to various classes of product. Having identified various paradigms for currently marketed products in the US including 510(k), Class III PMA, banked human tissue, Biologics License Application, and New Drug Application, the company needed to understand the corresponding regulatory pathways in Europe, and the regulatory requirements for bringing three of its products to market.
- Independently reviewing clinical trial data: A small biopharmaceutical company asked us to provide an independent review of data from a recently completed clinical trial. We were to provide a summary report for the company’s management and board of directors. Due to an upcoming board meeting, this request had a tight deadline of only six days from the initial transfer of data to submission of the final report.
- Chief Medical Officer support for listed biotech: A listed biotech company conducting Phase III clinical trials in an oncology indication needed Chief Medical Officer support for multiple clinical activities in Europe and the United States. We provided medical oversight of three ongoing clinical trials, in particular, providing medical oversight for a Phase I trial in preparation for a marketing application, a data and safety measurement board (DSMB) review of an ongoing Phase IIb clinical trial, and the launch of a Phase II clinical study in pancreatic cancer.
Selection of Clinical Development Case Studies:
Challenge: A venture capital-backed platform technology company wanted to develop its internal pipeline of therapeutic candidates, while allowing prospective partners to access the technology platform through selected R&D collaborations.
Solution: Our oncology consultant created a detailed timeline and gap analysis for the company’s initial IND filing and Phase 0-l clinical trial launch, and conducted the following tasks.
-
Reviewed relevant documents, including the technology, pre-existing corporate, scientific and clinical goals, and any preclinical data
-
Held interviews with key stakeholders including company founders, the CEO and the internal R&D team
-
Reviewed potential target cancer indications and establishing up-to-date standards of care
-
Reviewed relevant biomarkers to inform PK/PD activity and patient enrichment strategies
-
Prepared a clinical development plan including prioritization of target indications, design of Phase 0-lla clinical trials and proposal for clinical trial investigators and sites
-
Developed an interval-to-IND filing gap analysis.
Our consultant presented to the board and was retained as an ongoing medical advisor to the company. Further case studies regarding the work Alacrita performs in clinical development are available here.
Challenge: For a European pharmaceutical company, a highly-experienced Alacrita consultant with a background in endocrine and metabolic therapies acted as medical director for the registration of a new agent in a neuroendocrine indication in territories outside the USA. Alacrita was brought in by the client five months before the planned MAA submission date after the internal medical director and other key team members unexpectedly left the company and could not be replaced internally.
Solution: During the five-month period, the team finalized the study reports, wrote the overview and the SmPC for the submission as well as preparing an ODD application (which was granted). The team received numerous questions from the rapporteur and co rapporteur and the same team worked on the responses to questions, including new statistical analyses of the clinical data. The Alacrita medic represented the company in regulatory agency meetings and the product was successfully steered through regulatory approval. Following this, submissions were prepared for other non-US territories and the dossier was transitioned to the client’s commercial team.
Challenge: A listed biotech company conducting Phase III clinical trials in an oncology indication needed Chief Medical Officer support for multiple clinical activities in Europe and the United States.
Solution: We provided medical oversight of three ongoing clinical trials, in particular, providing medical oversight for a Phase I trial and preparing for:
- marketing application
- data and safety measurement board (DSMB) review of an ongoing Phase IIb clinical trial
- launch of a Phase II clinical study in pancreatic cancer.
Challenge: A US-based cancer vaccine company developing a peptide-based product was in clinical trials in its lead indication. Recent laboratory research had revealed the target antigens were also expressed on a number of other solid tumor types, and the company wanted to assess which of those indications were the most attractive for expanding the program.
Solution: Alacrita conducted desk research and physician interviews to identify the patient populations that could be most amenable to cancer vaccine therapy and clinical development. Findings suggested that the ideal clinical trial for a cancer vaccine enrolls a group of patients that have minimal residual disease, intact immune systems and at least 18 months of expected survival. We also found a clinical planning trade-off between patient disease stage (and consequent likelihood of response to a cancer vaccine), and anticipated clinical trial duration. We identified a number of possible clinical development options that met our client’s requirements, including an exploratory clinical trial that could be conducted rapidly to provide evidence of biological activity to help justify investment in a larger, randomized clinical program.
Challenge: A European pharmaceutical company required medical, clinical and technical expertise for radoipharmaceuticals, theranostic development and medical imaging. Alacrita was engaged to provide the necessary support..
Solution: The Alacrita team provided expertise that was not available within the client organization, and support for a portfolio of molecules spanning Phase I and Phase II clinical trials was critical to the timely execution of the development plan. Alacrita provided this support over a period of three years.
The following support was provided:
- Provided strategic and scientific expertise into radiopharmaceutical and theranostic development and medical imaging
- Helped design and develop protocols/studies with imaging as a primary or secondary endpoint
- Recommended, built consensus and then designed Phase II protocol for the first multi-center study which was designed to answer the FDA pre-IND questions.
- Critically reviewed and helped design all medical imaging assessments for oncology teams across four protocols
- Evaluated the program and ROI and recommended a companion diagnostic development pathway.
- Supported the evaluation and operational management of the imaging core labs (ICL) in studies where medical imaging was being used, including careful review and editing of Imaging Charters and Image acquisition guidelines.
- Acted as interim senior clinical development leader for a Phase I trial
- Provided input to and implemented the strategic direction and tactical execution of drug development trials
- Wrote the protocols and provide insight into all the imaging endpoints.
- Developed the concept of modified Ga RECIST, modified Ga PERCIST and volumetric assessments in the protocols to ensure the theranostic approach would be fully elucidated any abscopal effects would be seen.
- Provided scientific expertise, strategic and operational input to external innovation and due diligence activities as requested.
- Supported the regulatory teams in representing the company in regulatory agency and health authority relations including:
- Wrote the initial response to the FDA re the IND
- Supported and wrote some of the sections for the IND
- Developed and wrote a significant part of the questions to the FDA briefing book for the FDA to support the approach for a companion diagnostic
- Suggested the opportunity to meet with the FDA as part of a Critical Path for Innovation meeting (CPIM) and developed the document submitted to the FDA and slides for the possible meeting.
Challenge: A venture capital-backed platform technology company wanted to develop its internal pipeline of therapeutic oncology candidates, while allowing prospective partners to access the technology platform through selected R&D collaborations. Alacrita was asked to help create a detailed early clinical plan for the company's lead asset, to be used as a foundation piece of the business strategy.
Solution:
Our oncology consultant created a detailed timeline and gap analysis for the company’s initial IND filing and Phase 0-l clinical trial launch.
This involved:
- reviewing relevant documents, including the technology, pre-existing corporate, scientific, and clinical goals, and any preclinical data
- holding interviews with key stakeholders including company founders, the CEO and the internal R&D team
- reviewing potential target cancer indications and establishing up-to-date standards of care
- reviewing relevant biomarkers to inform PK/PD activity and patient enrichment strategies
- preparing a clinical development plan including prioritization of target indications, design of Phase 0-lla clinical trials and proposal for clinical trial investigators and sites
- developing an interval-to-IND filing gap analysis.
Recent Drug Development White Paper:
-
FDA Expedited Programs for Cancer Drug Development: Don’t Believe the Doubters
Approximately 40% of recently approved cancer drugs have gone through one of four established FDA expedited programs. These treatments account for billions of dollars in sales and help create a thriving, industry-wide pipeline. The FDA has also recently approved the first New Drug Application (NDA) under one of its new pilot programs intended to speed cancer drug review, leading to optimism that these programs will also help speed delivery of more drugs to patients. But there has also been fresh criticism about results of the Accelerated Approval pathway and whether post-market trials support approval of most of these drugs. Will concerns about overuse lead to curtailment of these programs? Or do these concerns just reflect the age-old tension between getting drugs approved faster and knowing more about them before they are approved? Have criteria for what makes a good candidate for an expedited program changed? Here, we review the latest developments with insights and comments from Dr. William (Bill) Slichenmyer, a partner at Alacrita and an oncologist with more than 20 years of experience in the biopharma industry.
Read More

