From preclinical strategy to hands-on clinical development support, Alacrita provides a range of product development services covering the full product life cycle. Our consultants average 20-30 years of industry experience, allowing them to draw on significant first-hand knowledge to help clients progress optimally, while navigating today's increasingly competitive, complex and rapidly-changing drug development environment.
Our core team leverages our Expert Network, which contains over 150 expert product development consultants who specialize in specific technologies and disease areas, across the below functional disciplines:
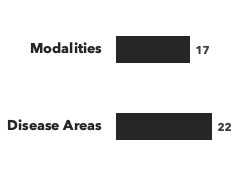
Number of consultants by education, and number of modalities/disease areas covered:
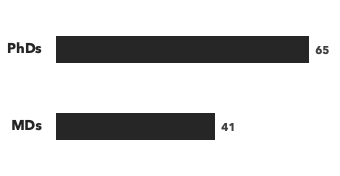
Contact us today to learn how our pharma & biotech consulting expertise can serve your project.
Contact us© 2009-24 Alacrita Holdings Limited | Pharma & Biotech Consulting
Alacrita Consulting
2 Royal College St
London, UK
NW1 0NH
Registered in England & Wales.
No. 10530608
One Broadway
Floor 14
Cambridge, MA 02142
Registered in Massachusetts.